Quality Assurance Services
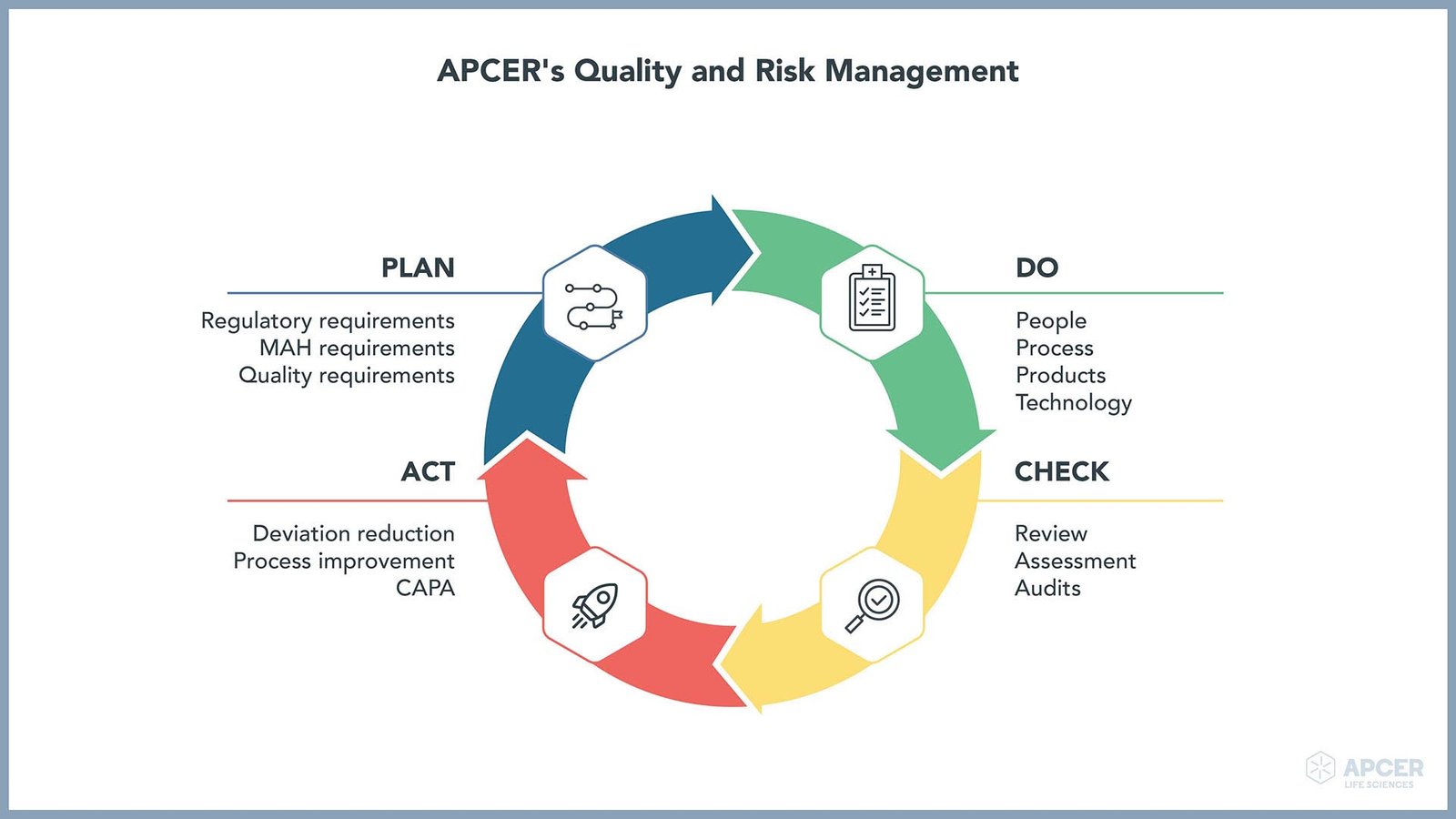
An effective quality management system (QMS) is essential for marketing authorization holders (MAHs) to meet unannounced and stringent regulatory inspections. Besides increasing complexity of clinical development and pharmacovigilance (PV), the regulatory requirements of various health authorities are also constantly updating and getting specific. To have a compliant QMS, MAHs need to ensure a robust and effective audit program and maintain routine QMS activities.
APCER provides effective and efficient Quality Assurance (QA) services to ensure compliance to regulatory requirements and industry standards, resulting in safe and effective use of medicinal products. Through trained and experienced quality auditors specializing in PV and clinical domain, APCER can provide expertise and an anytime inspection-ready environment.